6 Interesting Facts About Potassium in the Periodic Table
Potassium is a vital element in the periodic table, known for its intriguing properties and essential role in various biological processes. Sir Humphry Davy’s discovery of potassium in 1807 was a pivotal moment in the history of chemistry, particularly in the study of alkali metals. Let’s look at six fascinating facts that showcase potassium’s significance in both science and everyday life.
Potassium Basics
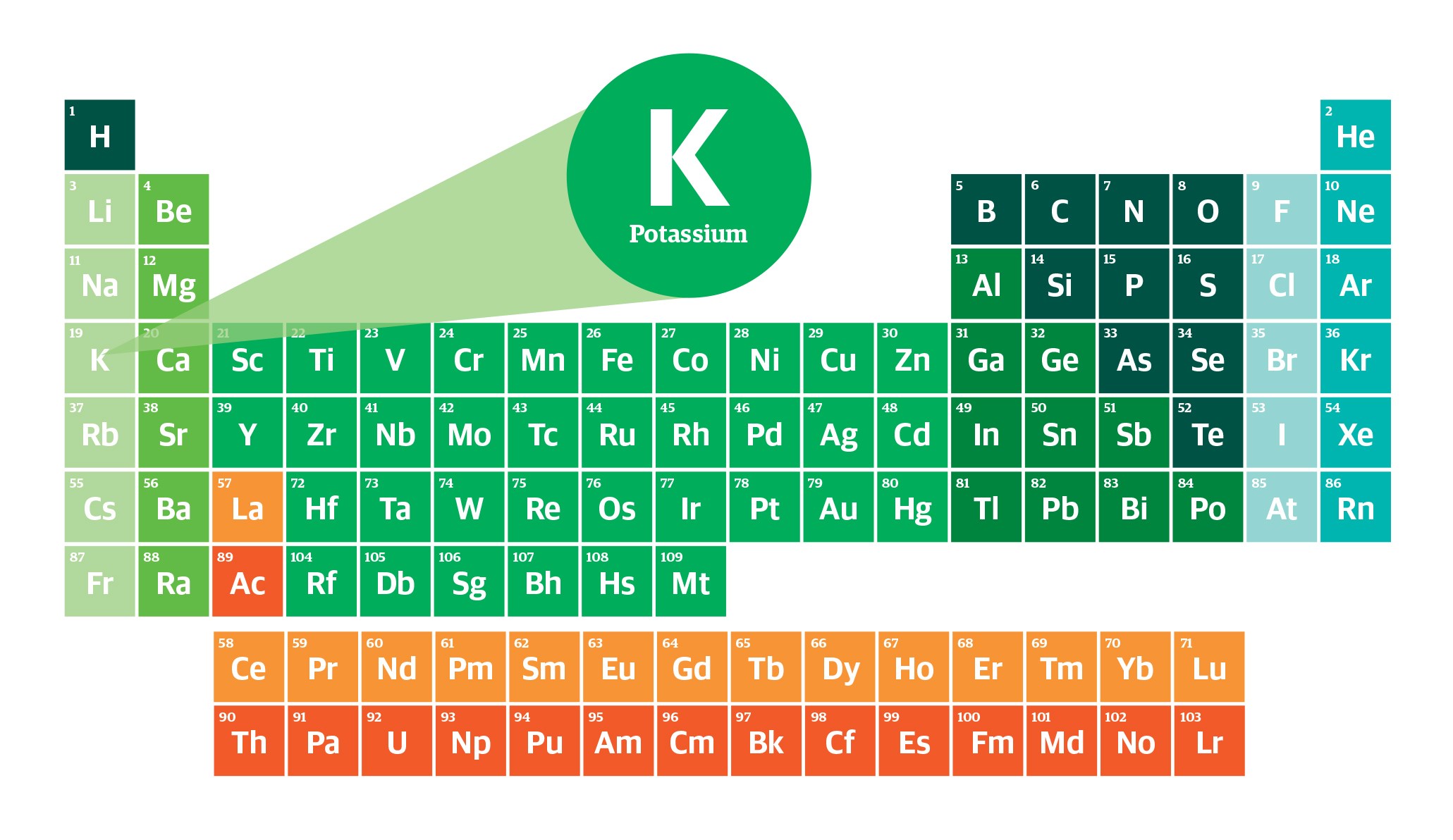
Potassium, symbolized as K on the periodic table, belongs to Group 1 and Period 4. It’s an alkali metal with an atomic number of 19 and an atomic weight of approximately 39.098 u. This element was first isolated by Sir Humphry Davy in 1807 through the electrolysis of molten potassium hydroxide. Here’s a deeper look into what makes potassium a standout element:
1. Abundant in Nature
Potassium is the seventh most abundant element in the Earth’s crust, making up about 2.6% of its mass. It’s primarily found in minerals like sylvite (KCl), carnallite (KMgCl3·6H2O), and langbeinite (K2Mg2(SO4)3), among others. These minerals are essential sources of potassium for various industrial and agricultural applications.
2. Reactive Nature
Potassium is highly reactive, especially with water. When exposed to water, potassium vigorously reacts to produce hydrogen gas and heat, a characteristic shared with other alkali metals. This reactivity arises from its single valence electron in the outermost shell, which it readily donates to achieve a stable electron configuration.
3.Handling Precautions – 6 Interesting Facts About Potassium in the Periodic Table
Due to its reactivity, potassium is stored under mineral oil to prevent reaction with moisture in the air. Handling potassium requires caution and expertise to avoid accidental ignition or explosions, particularly in laboratory settings.
4. Biological Importance – 6 Interesting Facts About Potassium in the Periodic Table
Potassium ions (K+) play a crucial role in cellular function and nerve transmission in living organisms. They are essential for maintaining proper heart rhythm, muscle contraction, and nerve function. The concentration of potassium ions inside and outside cells is tightly regulated to ensure these physiological processes function correctly.
Is kenza layli real ? World’s First Miss AI | Maya (mayathevoice.com)
5. Dietary Requirements – 6 Interesting Facts About Potassium in the Periodic Table
Humans require a regular intake of potassium through diet, with recommended daily amounts varying by age and health status. Potassium-rich foods include bananas, potatoes, spinach, and citrus fruits. A balanced diet helps maintain adequate potassium levels, crucial for overall health.
6. Flame Test Color
One distinctive feature of potassium is its characteristic flame color. When potassium salts are burned, they impart a lilac or pale violet hue to the flame. This phenomenon is utilized in flame tests to identify the presence of potassium in compounds and mixtures.
7. Isotopes and Applications
Potassium exhibits three naturally occurring isotopes: potassium-39, potassium-40, and potassium-41. Potassium-40, with a half-life of 1.25 billion years, undergoes radioactive decay to form argon-40. This decay process is used in potassium-argon dating to determine the age of rocks and geological formations.
8. Medical Applications – 6 Interesting Facts About Potassium in the Periodic Table
Radioactive potassium-40 isotopes are also employed in medical diagnostics, particularly in nuclear medicine imaging. They help visualize physiological processes within the body and diagnose certain medical conditions.
9. Industrial Uses
Beyond biological and medical applications, potassium compounds have various industrial uses. Potassium hydroxide (KOH) is crucial in the manufacture of soaps and detergents. Potassium nitrate (KNO3) finds use in fertilizers, explosives, and fireworks due to its oxidizing properties.
Conclusion – 6 Interesting Facts About Potassium in the Periodic Table
Potassium’s significance extends far beyond its position on the periodic table. From its role in biological functions to industrial applications and geological dating methods, potassium continues to intrigue scientists and benefit humanity in diverse ways. As we deepen our understanding of this essential element, its relevance in both natural and synthetic realms becomes increasingly apparent.
In summary, Humphry Davy’s discovery of potassium exemplified the experimental rigor and innovative techniques characteristic of early 19th-century chemistry, marking a significant milestone in the field of elemental chemistry and electrolytic processes. Davy’s discovery of potassium, along with his subsequent isolations of other alkali and alkaline earth metals, was instrumental in advancing our understanding of chemical elements and their characteristics. His work not only expanded knowledge in electrochemistry but also laid a solid foundation for further exploration into the properties and behaviors of metals.
This article provides an in-depth exploration of potassium’s key attributes, aiming to inform and engage readers with its multifaceted nature in chemistry and beyond. To read more kindly subscribe to our blog and Youtube channel.